Second Law of Thermodynamics
Efficiency
Early statements
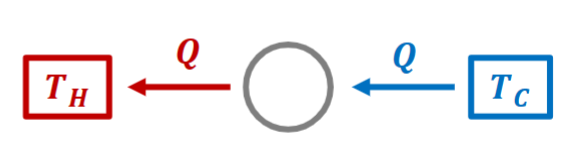
Clausius Statement
Created by Rudolf Juilius Emanuel Clausius
It is impossible to construct a device that operates in a cycle and produces no other effects than the transfer of heat from a cooler body to a hotter body.
Layman terms:
- Heat goes from hot to cold
- If you want heat to go from cold to hot, you need to do work.
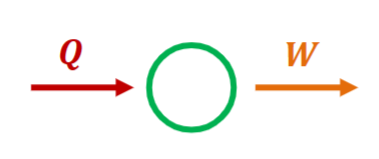
Kelvin-Planck Statement
Created by William Thomson, 1st Baron Kelvin and Max Karl Ernst Ludwig Planck
It is impossible to construct a device that operates in a cycle and produce work by exchanging heat with a single reservoir.
Layman terms:
- To produce work, you need a cold and a hot source.
- To produce work, you will lose/waste some heat.
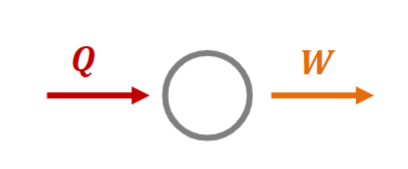
Proof
Let Clausius' statement be wrong.
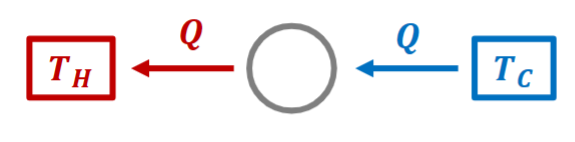
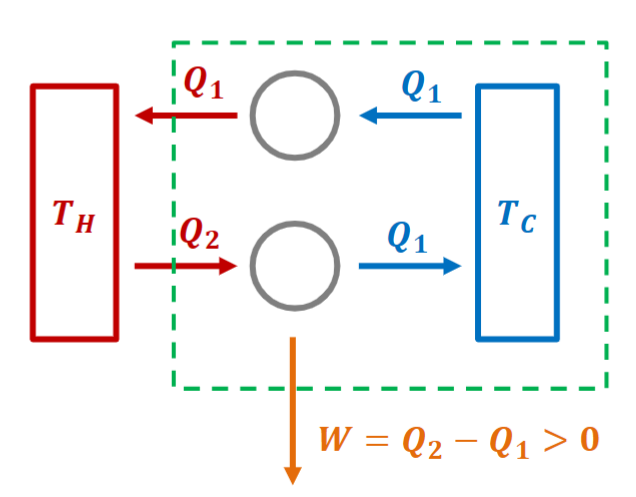
Thus, Kelvin-Plank statement is wrong.