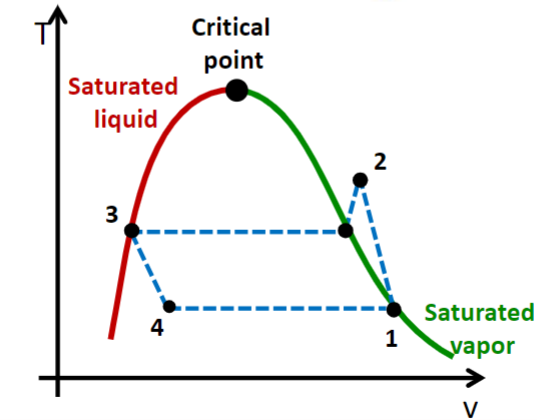
Vapor Compression Cycle
Working fluid:
- Fluid with Phase Changes
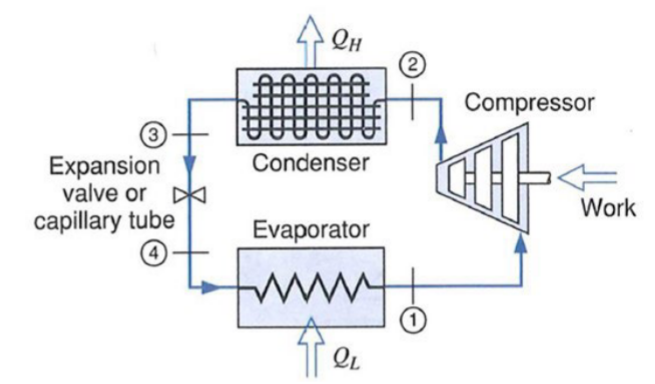
Details:
: Isentropic compression | |
: Constant Pressure | | - Phase change:
|
: adiabatic expansion (not isentropic!) | - Not reversible!
: Constant Pressure | |
Assumptions:
- State 1 = saturated vapor
- State 3 = saturated liquid
- Negligible kinetic energy
Refrigerants:
Early days:
- Ammonia (
) and sulfur dioxide ( ) - Highly toxic
CFC: Chlorofluorocarbons Banned
- Ex:
- Dichlorodifluoromethane (R-12): Original "Freon"
- Chlorodifluoromethane (R-22)
- Lead to depletion of ozone (due to Cl)
HFC: Hydrofluorocarbons
- Ex:
- Tetrafluoroethane (R-134a)
- Difluoromethane (R-32)
- Pentafluoroethane (R-125)
- No chlorine, but greenhouse gas >
Remark:
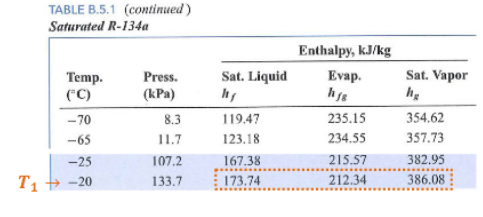
Example:
Conditions:
- Working fluid: R-134a
- Low temperature:
- High temperature:
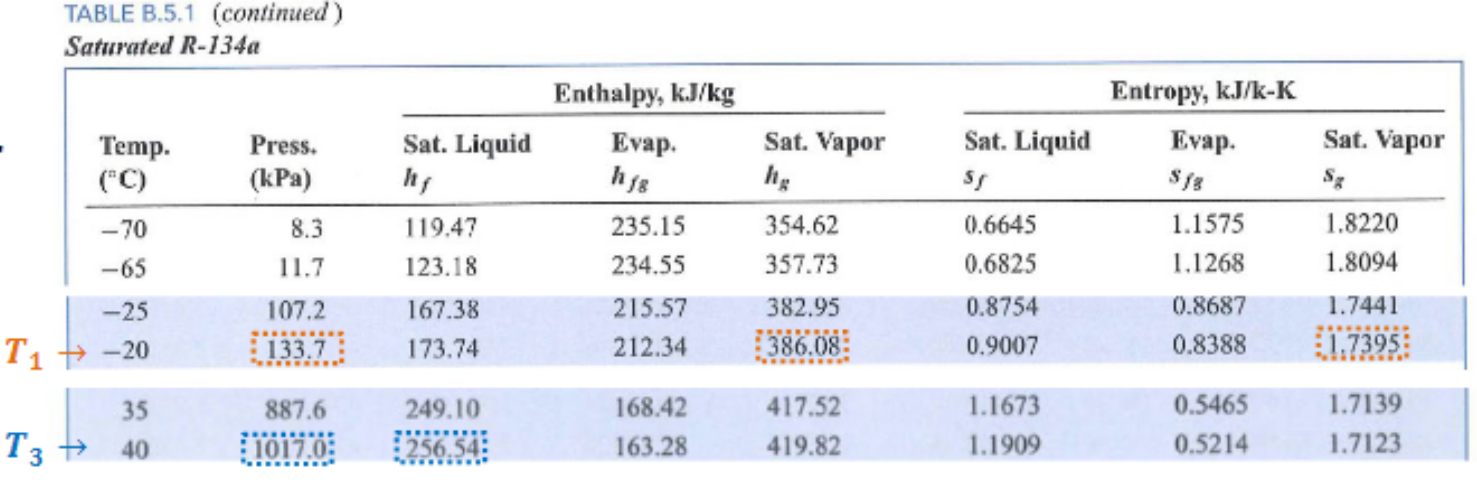
State 1:
- Saturated Vapor
State 3:
- Saturated Liquid
Processes:
Process
- Constant Pressure
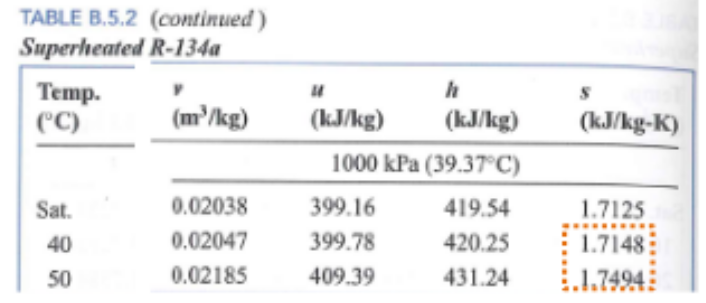
Thusand
Process
- Isentropic
- First Law for Constant Volume
Process
- First Law for Constant Volume
Process
- Adiabatic, no work
- First Law for Constant Volume
Thus:
Process
- Constant Pressure
- Phase Change
- First law for Constant Volume
Coefficient of Performance:
Back to process
- Adiabatic
- No work
Enthalpies:
- State 3: Saturated liquid
- State 4: Vapor + liquid
Entropies:
- State 3: Saturated liquid
- State 4: Vapor + liquid
Recall: Gibbs Equation
since .
Real Case:
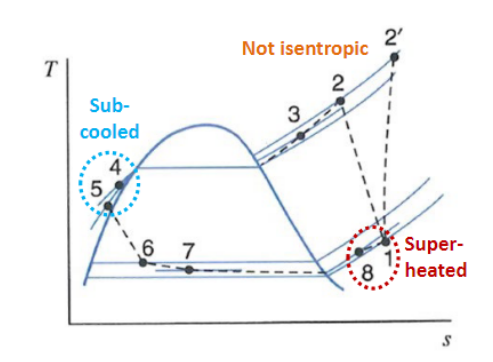
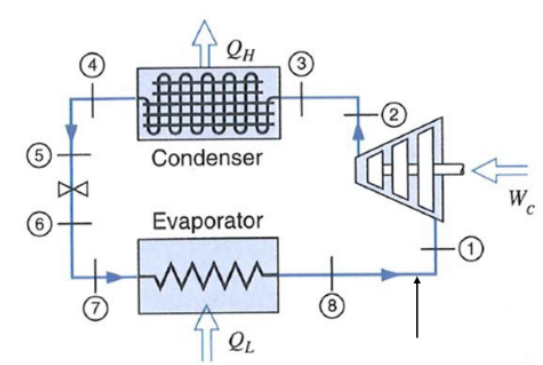
Piping causes pressure drops and heat losses.