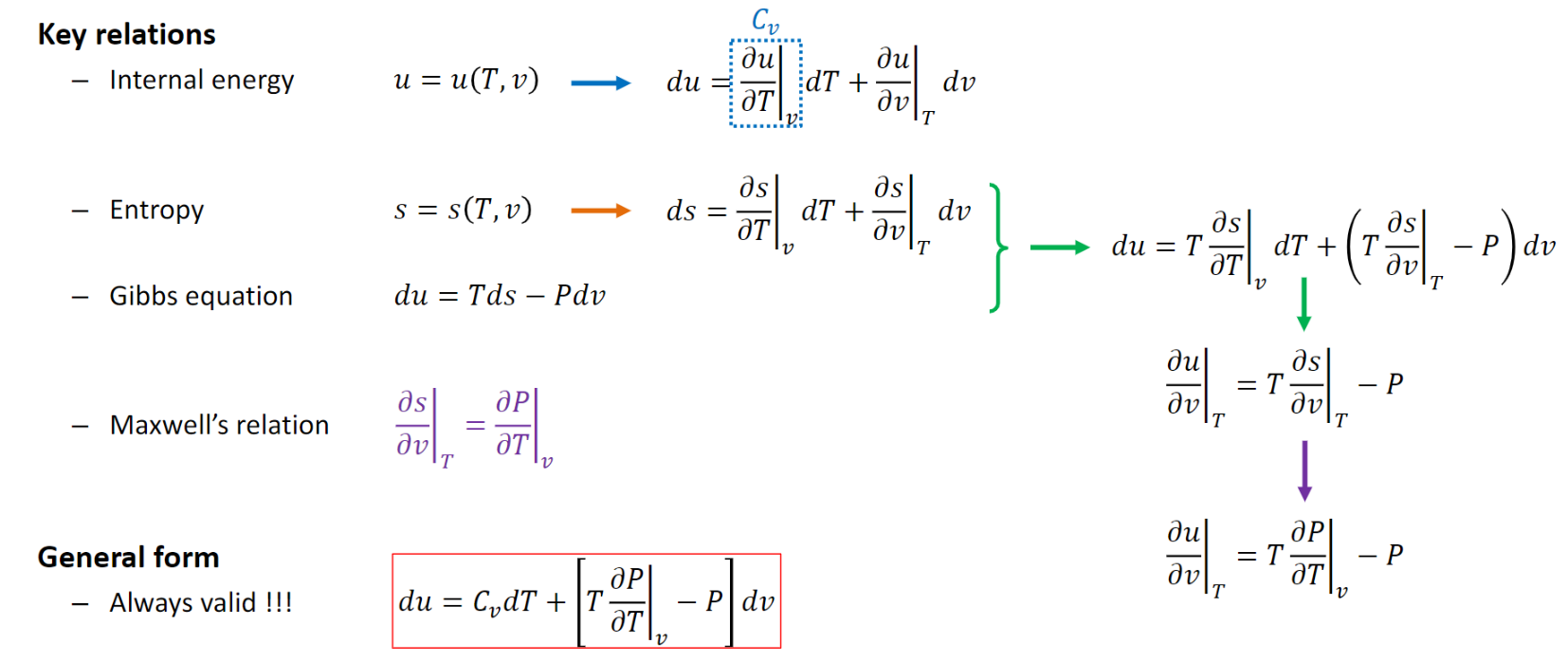
Thermodynamic Relations
Perfect Liquids/Solids:
- Incompressible:
- Small Specific Volume:
Heat Capacities:
Internal Energy:
Entropy:
From Internal Energy...
Ideal Gas:
Fully compressible:
Internal Energy/Enthalpy:
Heat Capacities:
Internal Energy:
Enthalpy:
Entropy:
From Internal Energy in terms of Specific Volume...
From Enthalpy in terms of Pressure...
Internal Energy: